Applications and Uses

Baking soda food coloring vinegar – The baking soda, vinegar, and food coloring reaction, a classic example of an acid-base reaction, offers a wealth of applications beyond a simple kitchen experiment. Its visual appeal and ease of execution make it a valuable tool in both educational and creative settings. The reaction’s predictable outcome and readily available materials ensure its suitability for a wide range of uses.
This visually striking reaction provides a captivating introduction to fundamental chemical concepts for young learners. The effervescence and color change are engaging and memorable, fostering curiosity and a deeper understanding of chemical processes.
Educational Applications, Baking soda food coloring vinegar
The baking soda and vinegar reaction serves as an excellent demonstration of several key scientific principles. In educational settings, it can be used to illustrate concepts such as acid-base reactions, gas production (carbon dioxide), and chemical changes. Teachers can adapt the experiment to different age groups, incorporating variations in complexity and focusing on specific learning objectives. For instance, older students could explore the quantitative aspects of the reaction, measuring the volume of gas produced or investigating the effect of different concentrations of reactants.
The reaction between baking soda, vinegar, and food coloring creates a classic science experiment demonstrating gas production. The vibrant color change is often enhanced using a strong, concentrated food coloring like Wilton orange food coloring , which provides a rich hue. This makes the resulting foam more visually appealing, illustrating the chemical reaction more effectively. The intensity of the orange color highlights the volume of gas produced from the baking soda and vinegar mixture.
Younger students can focus on the observable changes, like the fizzing and color change, while still grasping the fundamental concept of a chemical reaction.
Simple Science Experiments for Children
The reaction’s simplicity makes it ideal for children’s science experiments. A classic example is creating a “volcano” using a plastic bottle, baking soda, vinegar, and food coloring. The eruption of foamy colored liquid simulates a volcanic eruption, providing a fun and engaging learning experience. Variations include using different shaped containers to alter the appearance of the “eruption” or adding small toys to the mixture for added visual interest.
The experiment can be easily adapted to explore concepts like density and buoyancy by adding different objects to the mixture and observing their behavior. Safety precautions, such as adult supervision and the use of eye protection, are crucial.
Safety Precautions
While generally safe, certain precautions should be taken when conducting this experiment. Adult supervision is crucial, especially with younger children. Eye protection should be worn to prevent accidental splashes of the mixture. The reaction produces carbon dioxide, a gas that can displace oxygen in a confined space; therefore, it’s advisable to conduct the experiment in a well-ventilated area.
Finally, reminding participants not to ingest any of the materials is essential.
Creative Applications
Beyond simple science experiments, the baking soda and vinegar reaction can be utilized in creative projects. The foamy mixture can be used to create unique textures in art projects. For instance, the mixture can be used to paint on paper or fabric, resulting in a bubbly, textured effect. The color variations offered by different food colorings allow for a range of artistic expressions.
This provides a unique avenue for exploring art and science in a synergistic manner. The reaction can also be incorporated into other crafts, such as creating colorful, fizzy bath bombs.
Modifications to Achieve Different Results
The baking soda and vinegar reaction is highly adaptable. Several modifications can be implemented to achieve different outcomes.
The following modifications can significantly alter the reaction’s outcome and provide opportunities for further experimentation and learning:
- Varying the amount of reactants: Increasing the amount of baking soda or vinegar will increase the amount of gas produced and the intensity of the reaction. Decreasing the amount will result in a less vigorous reaction.
- Adding other ingredients: Incorporating dish soap creates a longer-lasting and more voluminous foam. Adding different types of food coloring will yield a variety of colors.
- Changing the temperature: A warmer solution will generally react more quickly than a cold one.
- Using different acids: While vinegar (acetic acid) is common, other weak acids, such as citric acid (found in lemon juice), can be substituted to observe variations in reaction speed and intensity.
Variations and Experimentation
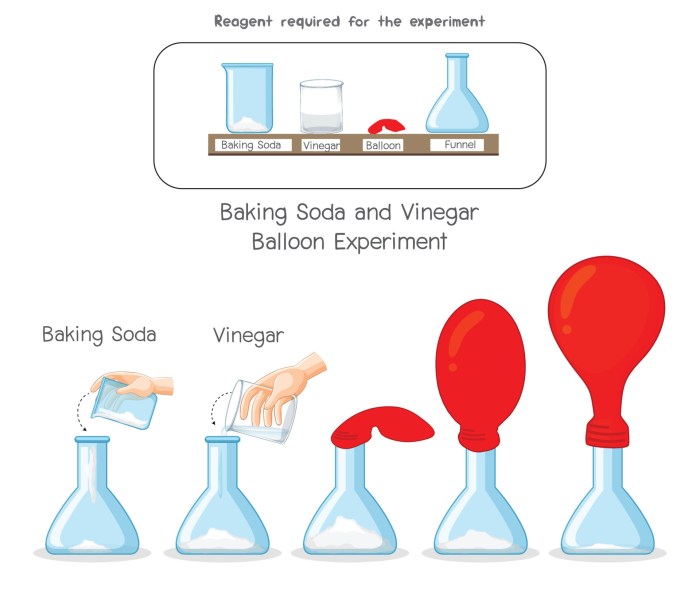
The classic baking soda and vinegar reaction, while seemingly simple, offers a surprising depth of experimental possibilities. By altering various parameters, we can observe significant changes in the reaction’s vigor and visual characteristics. This allows for a hands-on exploration of chemical kinetics and the impact of different variables on reaction rates.
Ratio of Baking Soda to Vinegar
Altering the ratio of baking soda (sodium bicarbonate) to vinegar (acetic acid) directly affects the reaction’s outcome. A higher concentration of baking soda, relative to vinegar, will initially produce a more vigorous reaction, generating a larger volume of carbon dioxide gas. However, once the limiting reactant (vinegar in this case) is consumed, the reaction will cease, regardless of the excess baking soda.
Conversely, using a larger quantity of vinegar relative to baking soda will result in a less intense, but potentially longer-lasting reaction, as the baking soda will be fully consumed before all the acetic acid.
Vinegar Concentration
The concentration of acetic acid in the vinegar significantly influences the reaction rate. Using a more concentrated vinegar solution (e.g., white vinegar versus apple cider vinegar, which has a lower acetic acid content) leads to a faster and more intense reaction. This is because a higher concentration of reactants increases the frequency of collisions between the baking soda and acetic acid molecules, thus accelerating the reaction.
Diluting the vinegar with water will slow the reaction down.
Impact of Added Liquids
Introducing liquids other than water can alter the reaction’s dynamics. For instance, adding a viscous liquid like honey or corn syrup might slow down the reaction due to increased viscosity, hindering the diffusion of reactants. Conversely, adding a less viscous liquid like alcohol (ethanol) might not drastically change the reaction rate, although it could slightly affect the overall volume of gas produced due to changes in the solution’s density.
Effect of Food Coloring Type
The type of food coloring used primarily affects the visual aspects of the experiment. Liquid food coloring typically disperses more readily throughout the solution, leading to a more even color distribution. Gel food coloring, due to its higher viscosity, might create more concentrated areas of color, resulting in a visually distinct pattern as the reaction progresses. However, the food coloring itself does not influence the chemical reaction between baking soda and vinegar.
Experimental Results Comparison
| Variable Tested | Baking Soda (grams) | Vinegar (milliliters) | Observation |
|---|---|---|---|
| Ratio: 1:1 | 10 | 10 | Moderate fizz, relatively fast reaction |
| Ratio: 2:1 | 20 | 10 | Very vigorous fizz, rapid reaction, some overflow |
| Vinegar Concentration: Diluted | 10 | 10 (50% water) | Slow fizz, less gas produced |
| Added Liquid: Honey | 10 | 10 | Slow, less vigorous reaction, gas bubbles trapped in honey |
Top FAQs: Baking Soda Food Coloring Vinegar
What happens if I use too much baking soda?
Using too much baking soda can result in a less vigorous reaction and potentially a less visually appealing outcome due to the excess baking soda not reacting completely.
Can I use different types of vinegar?
Yes, but the reaction rate might vary slightly depending on the concentration of acetic acid in the vinegar. White vinegar is generally recommended for consistency.
What are some safety precautions I should take?
Always supervise children during the experiment. Avoid contact with eyes and skin. Conduct the experiment in a well-ventilated area.
Can I use this reaction to make a volcano model?
Yes! This reaction is frequently used to create a model volcano, where the gas production simulates a volcanic eruption.
What if I don’t have liquid food coloring?
Gel food coloring can also be used, but it might dissolve more slowly and result in a slightly different visual effect.
